Nuclear Chemistry
This page explains the important terms and concepts in nuclear chemistry, including types of decay, nuclear reactions, and chain reactions.
Key Vocabulary
- Isotopes: Atoms of the same element with different numbers of neutrons. Example: Carbon-12 and Carbon-14.
- Nucleus: The center of an atom containing protons and neutrons.
- Radioactive Atom: An unstable atom that emits particles or energy as it decays.
Types of Radioactive Decay
- Alpha Decay (α): The atom emits 2 protons and 2 neutrons.
Example:238U → 234Th + a - Beta Decay (β): A neutron becomes a proton and emits an electron.
Example:14C → 14N + b⁻ - Gamma Decay (γ): High-energy rays are released.
Example:234Th* → 234Th + y - Nuclear Fission: A large nucleus splits into smaller ones.
Example:235U + n → 141Ba + 92Kr + 3n - Nuclear Fusion: Two small nuclei combine to form one larger nucleus.
Example:2H + 3H → 4He + n
Nuclear vs. Chemical Reactions
- Nuclear reactions involve the nucleus; chemical reactions involve electrons.
- Nuclear reactions release more energy than chemical reactions.
- Nuclear reactions are not affected by temperature or pressure.
- Chemical reactions form or break bonds between atoms.
Transmutation
Transmutation is when one element changes into another during a nuclear reaction.
Half-Life
The time it takes for half of a radioactive sample to decay.
- Example 1: Carbon-14 → Half-life: 5730 years
- Example 2: Iodine-131 → Half-life: 8 days
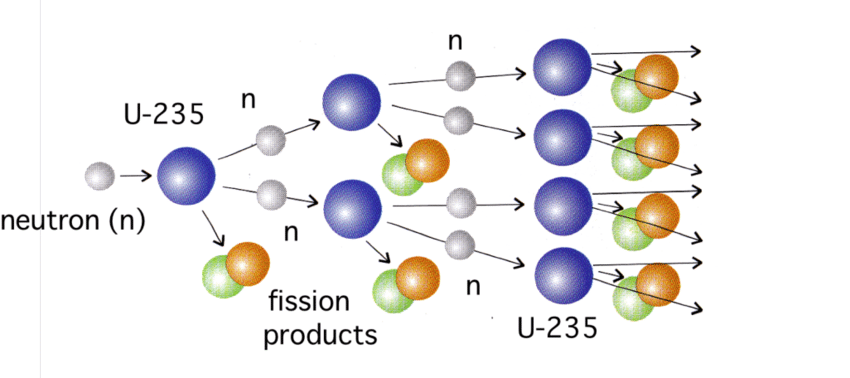
Nuclear Chain Reactions
In a chain reaction, released neutrons from fission trigger more fission reactions. This process can continue repeatedly.